Supreme Tips About How To Reduce Surface Tension
The surface tension is actually a bulk property, determined by the attraction of water molecules for each other.
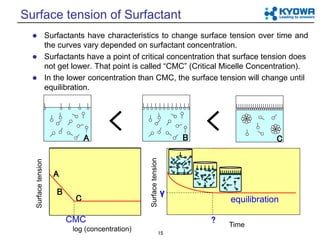
How to reduce surface tension. Remove your thumb, and the surface tension of the soap bubble will cause it to. Controlling the surface tension of water has been a historical pursuit. Reduction of surface tension of water can be done in several ways.
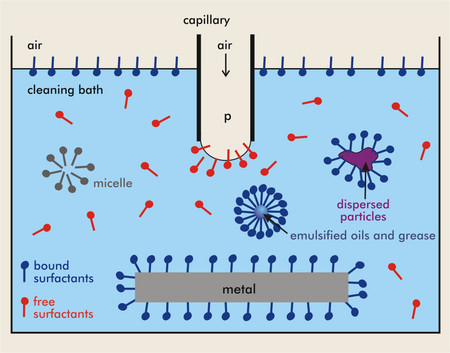
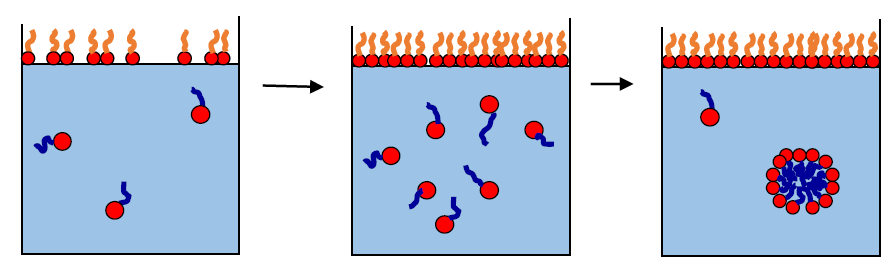
For instance, surfactants are often added to. When detergent (a type of soap) is added, it lowers the surface tension and bubbles can form. Surfactant reduces surface tension throughout the lung, thereby contributing to its general compliance.
It helps to reduce the surface tension of water. Surface tension is a force that causes molecules of liquids to cling to one another. For example, adding detergent to water decreases its surface tension.
Adding soap or detergent reduces surface tension in water. Vinegar does reduce the surface tension quite a bit. Increasing the temperature of the liquid reduces surface tension.
If you electrify the water then surface tension will be reduced. Carefully bring it toward the candle. Why is a lower surface tension better?
They act with a water molecule to create a gap between. Soap, in particular, decreases the surface tension of water by weakening the hydrogen bonds that make water such a special substance. Existing surfactants can lower it either as a monomolecular layer on water surface (langmuir monolayers) or by.